
Charco
As the Product Manager for CUE1, a wearable medical device for Parkinson’s patients, I led its entire lifecycle from prototype to production before transitioning into the Regional Manager role. This journey culminated in landmark achievements, including a $10 million seed funding round and an iF Design Award.
Understanding the science
Inspired by the pioneering neurologist Jean-Martin Charcot, CUE1 leverages focused vibration to ease rigidity and improve mobility for Parkinson’s patients. Charcot’s experiments in the 19th century revealed that tailored vibrations could temporarily reduce stiffness and enhance gait — a principle we reimagined in a modern, wearable format.
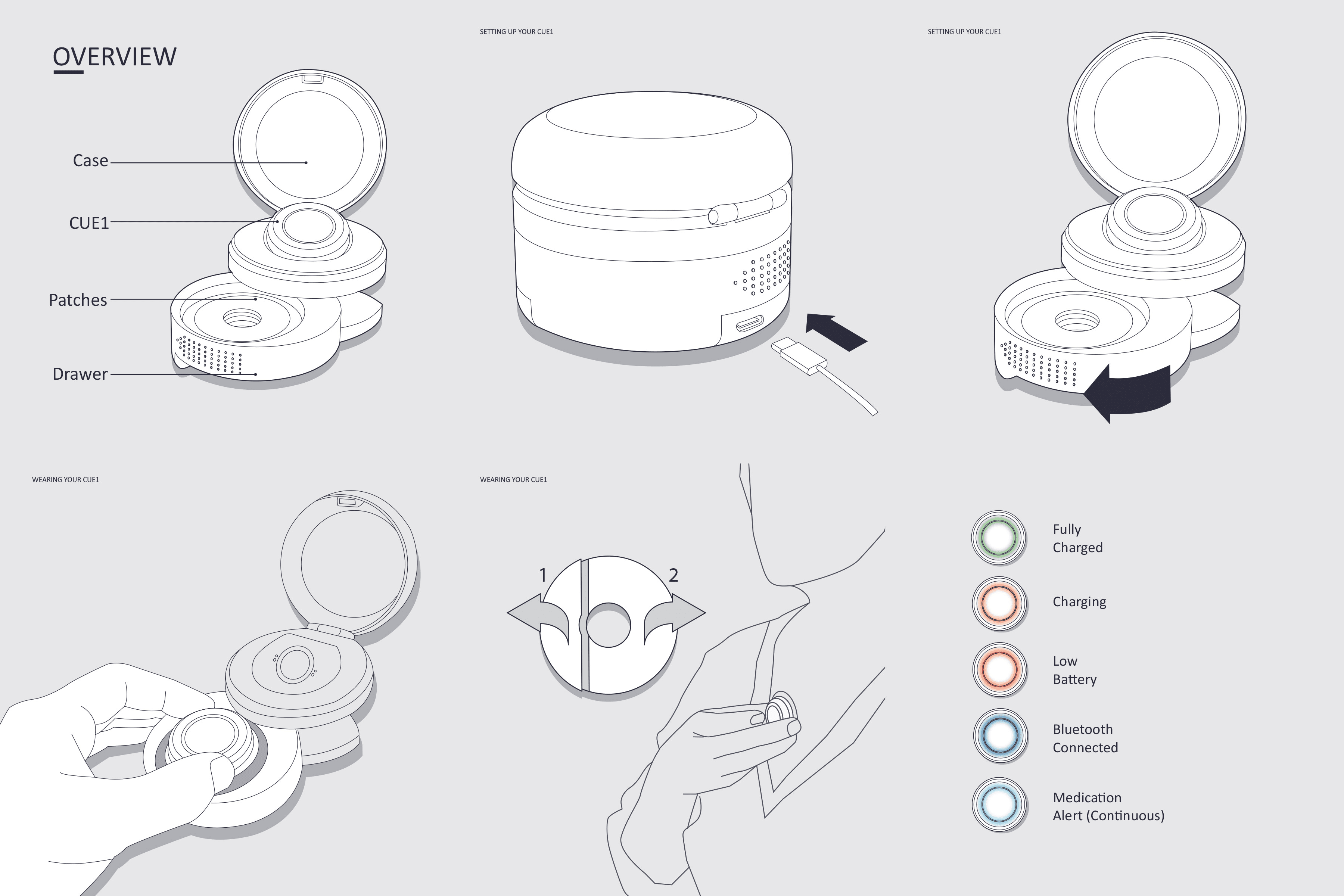
Shaping an approachable medical device
When I joined Charco, CUE1 was an early-stage prototype. My mission was to transform it into a production-ready, user-friendly device. Designing for Parkinson’s patients meant prioritizing simplicity, safety, and intuitiveness. By conducting interviews and home visits across the UK, we gathered rich insights into daily challenges and emotional responses, informing every design iteration.

Bridging device and app experience
The companion app was more than just a controller - it was a daily support system. We aimed to create a seamless extension of the device, allowing users to personalize vibration settings and track their progress. Balancing comprehensive data collection with ease of use was key, as many users were not tech-savvy. My role involved making critical UX decisions to remove friction and build confidence in both the hardware and the software.

Validating through real-world testing
Before formal clinical trials, I led three pivotal user studies and co-authored the related research reports, bridging clinical rigor with real-life insights.
Charco-Stappone User Testing: In this early UK-Austria collaboration, we combined CUE1 with insole sensor technology to objectively measure gait improvements in 5 Parkinson’s patients. The study provided foundational data on peak heel pressure, double support time, and gait symmetry, confirming early efficacy and informing future trial designs.
MDS-UPDRS & Usability Study: With 14 participants across various disease stages, we achieved a clinically significant mean improvement of 9.3 points in motor scores, along with better task performance. This study validated the device’s core therapeutic benefits and revealed critical usability improvements for both hardware and app.
Beta CUE1 Long-Term Home Study: 8 patients used the device daily for over 13 months, reporting improvements in gait, speed, stiffness, tremor, and confidence. Their diaries and feedback directly shaped product refinements, personalized stimulation settings, and long-term usability enhancements.
Together, these studies ensured that CUE1 met strict clinical standards while staying practical and empowering in real-world use.

Collaborating across borders
Beyond internal R&D, I orchestrated collaborations with CROs, manufacturing partners, testing labs, and hospitals. As I grew into the Regional Manager role, I oversaw the establishment of Charco’s Korean entity, managed local regulatory approvals, and led strategic partnerships. This experience honed my ability to manage complex, cross-functional projects on a global scale.

Driving impact and growth
By merging clinical rigor with user empathy, we delivered a device that not only met medical standards but also felt empowering to patients. Our efforts helped secure over $10 million in seed funding and an iF Design Award, validating our vision and design approach. Personally, I evolved into a confident leader capable of navigating both product strategy and regional business growth in an international setting.


